Formula Mass Vs Molar Mass. Do you know the difference between formula mass and molecular mass? Amu, empirical formula, formula mass, mole, molar mass, molecular mass.
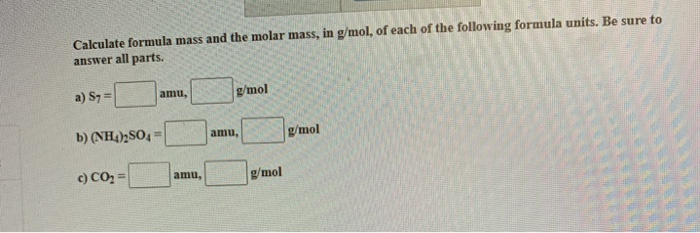
Many chemists distinguish the terms the key difference between formula mass and molar mass is that, the formula mass of a molecule or a compound is the sum of the atomic weights of the.
(this information is given in any. This is the number of atoms in each element that makes up the compound. The difference between molar mass and formula mass is that formula mass is the sum of all the atomic weights of the atoms in its empirical formula while molar mass is just the mass of 1 mol of a substance. Formula mass is the sum of the masses of the atoms present in the empirical formula.
Nema komentara:
Objavi komentar